| Using Marine Resources. Pollution. |
|
Petroleum death |
|
|
|
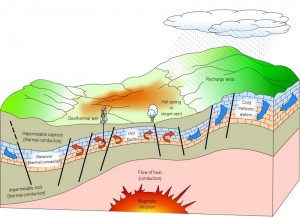
Geotermal sources |
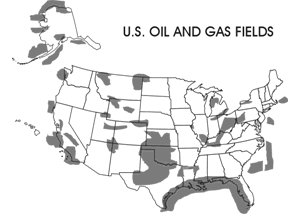
Petroleum |
Oolite beach - OOLITE BEACH, GREAT SALT LAKE UTAH. Oolites are concentric, sand sized concretions of mineral matter, in this case calcium carbonate. The mineral rich waters of the Great Salt Lake have created these oolites along the north shore of Antelope Island by agitating grains within the surf zone. As the grains move, they are coated with calcium carbonate - layer by layer, until they are too big to be moved by the currents. Notice the rounding of the grains and the well sorted nature of this sample. Oolites are normally associated with beaches |
|
CORAL PINK SAND DUNES STATE PARK UTAH. |
Oolitic sand is a common category of sand used by aquarists. The oolitic term describes the formation and type of sand. This sand is generally very small, fine, and round sand. Numerous hobbyists have used this sand in their tanks for many years, while others are still unaware of its existence. Some hobbyists argue against the use of this sand for various reasons, including; possible pollutants and contaminants, origination may not be of a marine source, potential problems for pH and alkalinity stabilization. Advocates of these sands claim they have: buffering capabilities, increased surface area for filtration purposes, low costs, and are composed of the same elements as high grade reef substrates. The purpose of this experiment was to test three common oolitic sand types for major element levels. These sand types were Great Salt Lake sand, the CaribSea Oolitic sand product, and the Southdown play sand found in Home Depots back east. The Southdown sand sampled was actually an OldCastle brand. Our supplier of this sand claims it is the brand and supplier to the Southdown play sand distributors. |
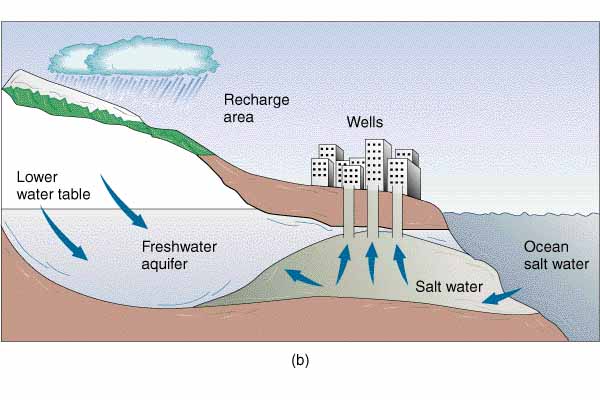
Excessive removal of water may reduce the pressure, so that salt water moves into the aquifer |
|
Northern fur seal pup entangled in fishing net |
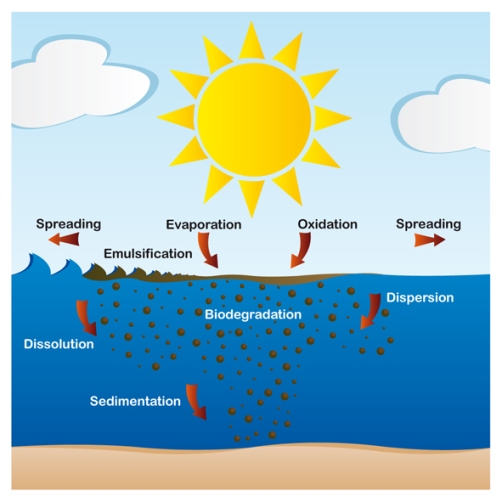
The fate of oil spilled at sea. |
Oil Spreading (oil slick) - Spreading is rarely uniform and large variations in the thickness of the oil are typical. After a few hours the slick will begin to break up and, because of winds, wave action and water turbulence, will then form narrow bands or windrows parallel to the wind direction. The rate at which the oil spreads is also determined by the prevailing conditions such as temperature, water currents, tidal streams and wind speeds. The more severe the conditions, the more rapid the spreading and breaking up of the oil. |
|
water -in - oil emulsion. The formation of these emulsions causes the volume of pollutant to increase between three and four times. This slows and delays other processes which would allow the oil to dissipate.Oils with an asphaltene content greater than 0.5% tend to form stable emulsions which may persist for many months after the initial spill has occurred. Those oils containing a lower percentage of asphaltenes are less likely to form emulsions and are more likely to disperse. Emulsions may separate into oil and water again if heated by sunlight under calm conditions or when stranded on shorelines. |
oil Sedimentation/Sinking. Oil stranded on sandy shorelines often becomes mixed with sand and other sediments. If this mixture is subsequently washed off the beach back into the sea it may then sink. In addition, if the oil catches fire after it has been spilled, the residues that sometimes form can be sufficiently dense to sink. |
Tarballs - Tarballs, the little, dark-colored pieces of oil that stick to our feet when we go to the beach, are actually remnants of oil spills. When crude oil (or a heavier refined product) floats on the ocean surface, its physical characteristics change. During the first few hours of a spill, the oil spreads into a thin slick. Winds and waves tear the slick into smaller patches that are scattered over a much wider area. Various physical, chemical, and biological processes change the appearance of the oil. These processes are generally called "weathering." |
|
|
Rachel Carson 1907-1964 |
Rachel Carson |
|
Marine oil polution |
|
|
RESOURCES AND POLLUTION
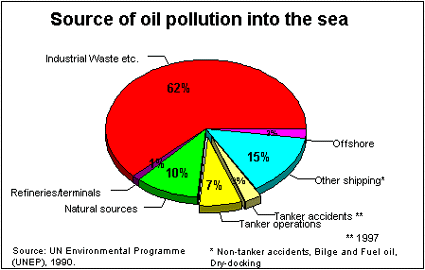
Sources of ocean oil pollution
Pollution can be a side effect of resource extraction and use.
Characteristics of a Pollutant (quantity and toxicity)
Biodegradable
A pollutant causes damage by interfering directly or indirectly with the biochemical processes of an organism.
Oil
Oil is a natural part of the marine environment. Oil seeps have been leaking large quantities of oil into the sea for millions of years. The amount of oil entering the ocean has increased greatly in recent years because of marine transportation of petroleum products, offshore drilling, nearshore refining, and street runoff carrying waste oil from automobiles.
Spills of crude oil
Spills of refined oil
Spills of crude oil are generally larger in volume and more frequent than spills of refined oil. Given equal volumes, refined or used oil is more damaging than crude oil to the marine environment.
Source: International Oil Spill Database ¢ Large Marine Oil Spills since 1960.
Heavy Metals ans Synthetic Organic Chemicals.chlorinated hydrocarbons
Biological amplication
Synthetic organic chemicals and heavy metals are persistent environmental poisons and can sometimes be concentrated by biological through food chains.
High level of toxic chemicals in fish livers, contamination by PCBs or pesticides, oxygen depletion, areas in which more than one-third of acreade is closed to commercial shellfish harvest.
Rachel Carson, author of the influential 1962 book SILENT SPRING about synthetic pesticides.
Synthetic organic chemicals and heavy metals are persistent environmental poisons and can sometimes be concentrated by biological amplication through food chains.
Eutrophication -
Uncontrolled algan growth through articially high nutrient levels ¢ Eutrophication ¢ can be gangerous to organisms and community diversity.
Solid Waste
Sewage sludge (sewage sediments)
The Costs of Pollution
ATMOSPHERIC CHANGES
The Ocean and atmosphere are extension of each other, and human activity has changed the atmosphere as it has changed the ocean.
Ozone Layer Depletion
Chlorofluorocarbons (CFCs)
The seazonal ozone ōholeö
The depletion of stratospheric ozone
Depletion of the atmosphereÆs ozone layer exposes surface organisms to higher levels of ultraviolet radiation and, consequently, to a greater risk of carner.
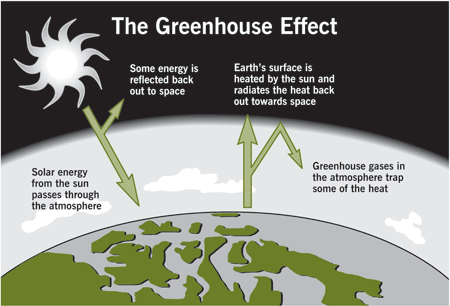
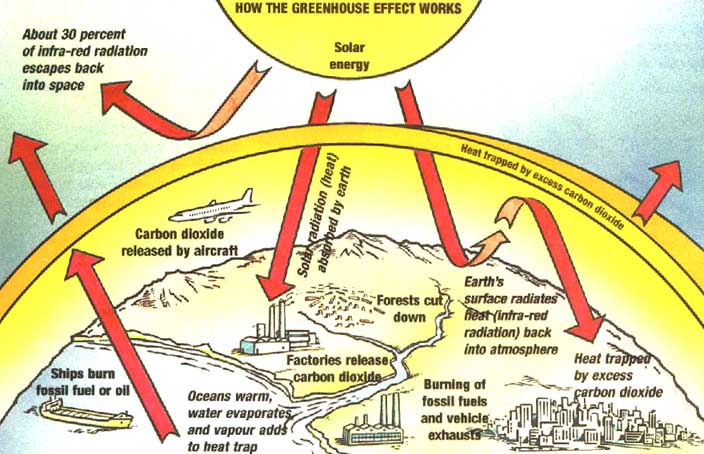
Global Warming
Greenhouse effect
Greenhouse gases
Ionizing rediation
Changes in the composition of the atmosphere can influence global climate, ocean temperatures, and sea level. Experts estimate that at least half of recent global warming is anthropogenic .